Direct-acting antiviral hepatitis C virus (HCV) therapies is one of the greatest medical advances in decades and has led to a major reversal in liver-related mortality. Yet despite this, treatment uptake in Australia declined rapidly between 2016 and 2020. While improving HCV treatment uptake is a key aim of global, national and state-based HCV strategies, this can’t be done without removing barriers to testing which exist in the current method of testing. Currently, testing and diagnosis is a long and drawn-out process requiring multiple visits to a health practitioner. An alternative approach is needed to enhance HCV testing and treatment in Australia.
ETHOS ENGAGE consists of blitz style HCV testing, utilising the Cepheid GeneXpert machine at drug and alcohol settings in Australia.
ETHOS ENGAGE has been enrolling participants since 2018 in periods of recruitment, Wave 1 (May 2018 – September 2019, 25 sites), Wave 2 (November 2019 – June 2021, 21 sites) and Wave 3 (May 2023 – January 2025, 28 sites). The Wave 4 period of recruitment is anticipated to commence in April 2025.
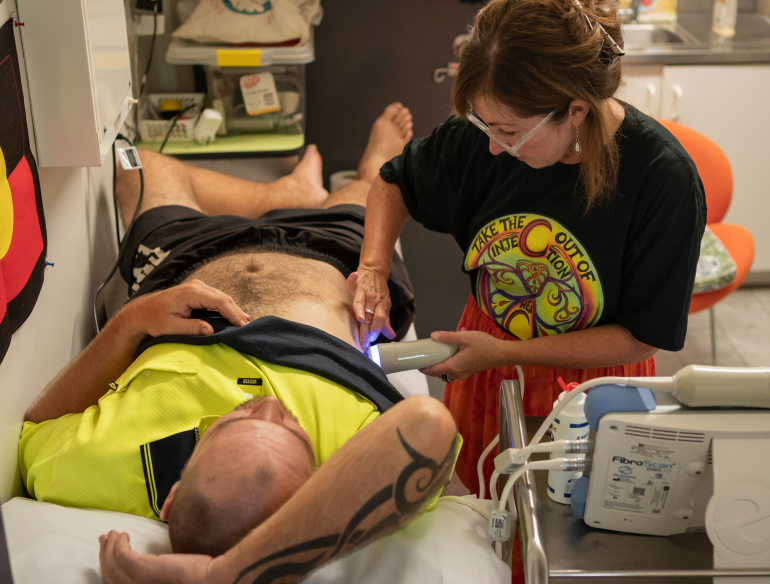
ETHOS ENGAGE provides participating sites access to fast, effective, TGA approved finger-stick HCV testing with results available for service clients in 1 hour. The project allows us to investigate the impact and benefits of this.
ETHOS ENGAGE is an observational cohort study collecting demographic, behavioural and clinical data among people who inject drugs. Participants are recruited from drug and alcohol services and Needle and Syringe Programs by peer support workers. All participants consent to take part in the study, complete a behavioural survey, have a finger-stick point-of-care test using the Xpert HCV Viral Load assay, have a FibroScan® to assess liver fibrosis and have a clinical consultation with a staff member at the site.
To date ETHOS ENGAGE has tested 3,962 people for HCV across 32 sites nationally
This project will help to better understand the potential impact of point-of-care HCV RNA testing, financial incentives, and same-day treatment among people who inject drugs. Information from patient, provider, and policy makers will help to understand facilitators and barriers to testing scale-up. Health economic evaluation of this intervention will inform future implementation. The cohort study with linkage of survey data will be invaluable in evaluating a range of long-term health outcomes among people who inject drugs. The project is expected to facilitate the translation of research outcomes to practice and policy, including informing national and international clinical guidelines, health service implementation, hepatitis strategies, and health policy.
Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM); The Australian Injecting and Illicit Drug Users League (AIVL); The Centre for Social Research in Health, UNSW Sydney; Canberra Alliance for Harm Minimisation and Advocacy (CAHMA); Cepheid; Harm Reduction WA; Hepatitis Australia; Hepatitis NSW; Hepatitis SA; Hepatitis ACT; Hunter New England Local Health District; Illawarra Shoalhaven Local Health District; Metro North Hospital and Health Service; Mid North Coast Local Health District; ; Nepean Blue Mountains Local Health District; Next Step Drug and Alcohol Services WA; Northern New South Wales Local Health District; Northern Sydney Local Health District, NSW Health; NSW Users and AIDS Association (NUAA); Queensland Government; NSW Health; NSW Users and AIDS Association (NUAA); Queensland Government; Queensland Injectors Health Network (QuIHN); SA Department of Health; South East Sydney Local Health District; South West Sydney Local Health District; Western Australia Department of Health; Western Sydney Local Health District.
NHMRC Partnership Grants, 1103165 (2016-2020) and (2022-2026); NSW Health.