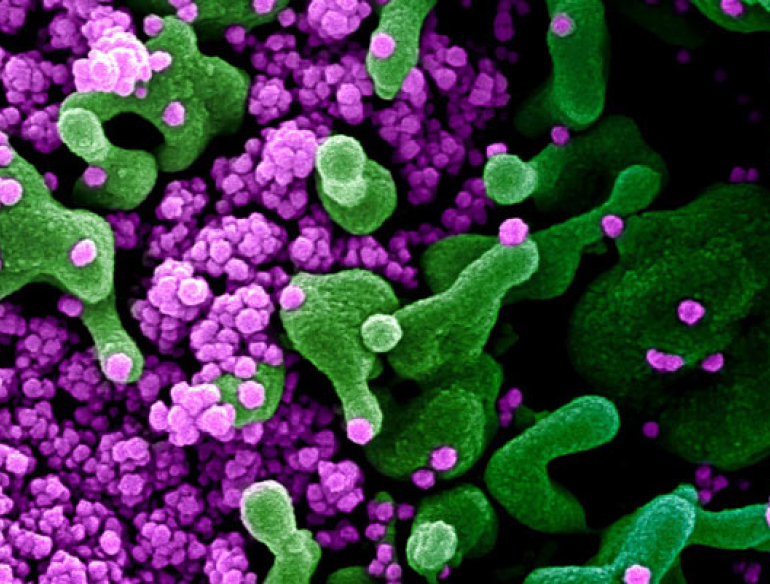
The COVID-19 pandemic requires an urgent response including a strong focus on effective immunotherapies, which boost the body’s immune response to the virus. One of the body’s immune response to infection is to produce antibodies, which fight the infection. Our researchers are looking for ways to harness these antibodies produced by recovered COVID-19 patients, to develop treatments for patients with active, progressive COVID-19 infection.
We have identified antiviral immunotherapy which uses ‘neutralising antibodies’ which defend the body’s cells against SARS-CoV-2 infection as a potential therapy. This type of therapy is likely to be safe and have a rapid pathway from the laboratory to a therapeutic products. One approach we are pursuing is using the plasma that contains antibodies from patients who have recovered from COVID-19, to purify a product called ‘hyperimmune globulin’.
We will identify patients who are recovering from COVID-19 infection and have a high concentration of neutralising antibodies. We will use these as a source of plasma to create a test batch of hyperimmune globulin which will be used to boost the body’s immune response to SARS-CoV-2. The plasma will then be used in human clinical trials initially in patients with asymptomatic or mildly symptomatic COVID-19 infection.
The clinical trial will provide data about dose and safety. The hyperimmune globulin should boost the levels of neutralising antibodies following infusion. If this is safe in those with mild infection, we will do further trials in larger numbers to sicker patients to see if this treatment prevents severe infection or speeds up their recovery.
Through our collaborations and expertise that spans the laboratory through to clinical trial design and conduct, we will be able to ensure rapid, large-scale evaluation of these approaches and, if these are promising, rapid production of the resulting therapy.
- St Vincent's Clinical School, UNSW
- Industry partner